CEPROTIN to treat acute purpura
fulminans and venous thrombosis
STUDY DESIGN:1
CEPROTIN was studied in a multi-center, open-label, non-randomized study in 3 parts which evaluated the safety and efficacy of CEPROTIN in patients with severe congenital protein C deficiency for the (on-demand) treatment of acute thrombotic episodes, such as purpura fulminans (PF), warfarin-induced skin necrosis (WISN) and other thromboembolic events, and for short-term or long-term prophylaxis.
Eighteen patients (9 male and 9 female), ages ranging from 0 (newborn) to 25.7 years participated in this study. The clinical endpoint of the study was to assess whether episodes of PF and/or other thromboembolic events were treated effectively, effectively with complications, or not treated effectively. Primary efficacy ratings of PF from the pivotal study was compared to the historical controls. Inadequate data was available for treatment of WISN.
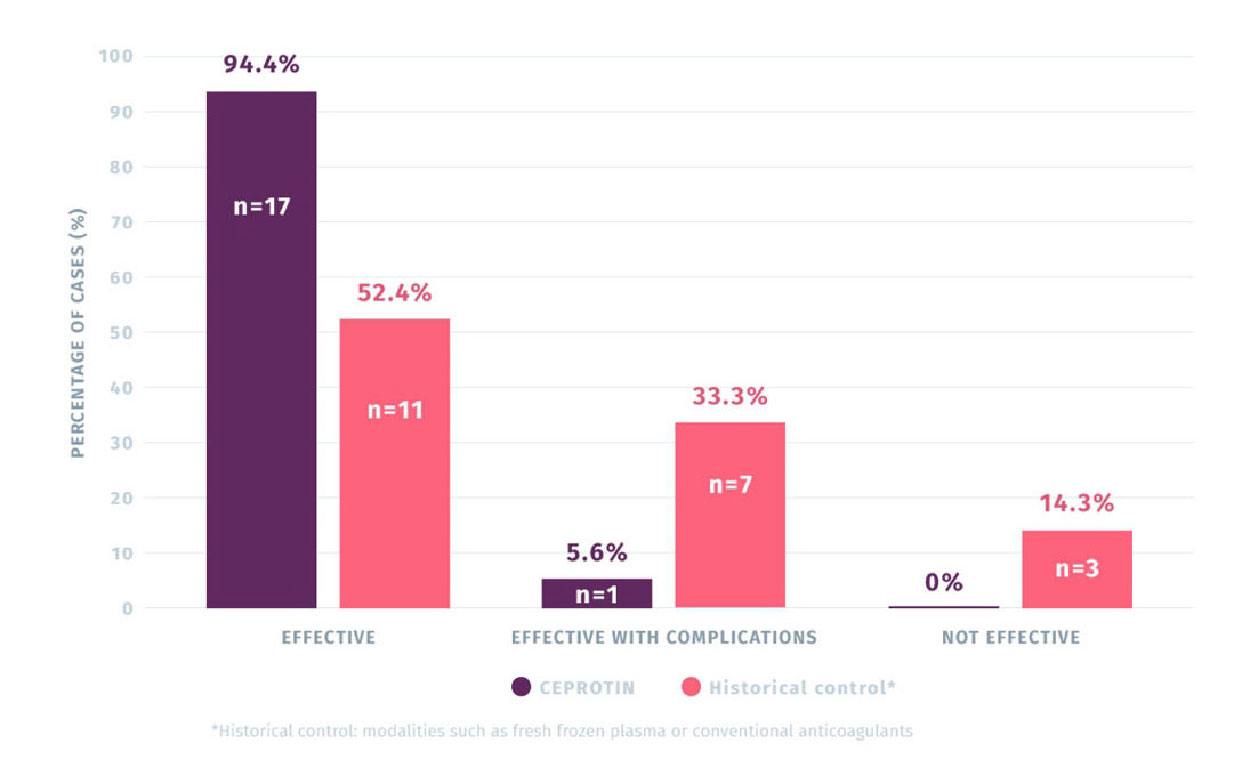
94.4% (N=17) OF CEPROTIN TREATMENTS WERE RATED AS EFFECTIVE IN
TREATING PURPURA FULMINANS (PF) COMPARED TO 52.4% (N=11) OF THE
HISTORICAL CONTROLS1,2
Effective:2
- The final treatment modality was effective and the patient was discharged on a stable anti-coagulation regimen
- Ineffective treatment modalities were disregarded, because in the historical control they could have been applied before the proper diagnosis of protein C (PC) deficiency was available (e. g. antibiotics for presumed sepsis)
Effective with complications:2
- The final treatment modality was effective (possibly with complications) and
- The patient was discharged on a stable anti-coagulation regimen, but ≥1 treatment modality was effective with complications
Not effective:2
- The final treatment modality was ineffective and/or
- The patient was not discharged on a stable anti-coagulation regimen
The primary efficacy rating was taken from 18 episodes of PF treated with CEPROTIN compared to 21 episodes of PF in the historical control group.
Primary endpoint efficacy rating of CEPROTIN for the treatment of PF vs. historical control group
(N=39 episodes)1
- Comparison of treatment efficacy revealed that episodes treated with CEPROTIN (n=18) was perceived as more effective than the historical treatments used to treat 21 episodes2
- The number of PF episodes deemed as ‘effective with complications’ were much smaller with CEPROTIN (5.6%, n=1) versus historical controls (33.3%, n=7).
-
Retrospective Analysis:1
A retrospective study to capture dosing information and treatment outcome data in patients with severe congenital protein C deficiency who were treated with CEPROTIN under an emergency use investigational new drug (IND) was also conducted.
Eleven patients (6 male and 5 female), ages ranging from 2.1 to 23.8 years participated in this study.
There were 28 acute episodes of purpura fulminans (PF) / warfarin-induced skin necrosis (WISN) and vascular thrombus reported in which time to resolution ranged from 0 to 46 days. The treatment outcome for these episodes was rated effective in all cases except one.
SAFETY RESULTS:
11 patients were treated with CEPROTIN for acute thrombotic events of which:2
- 7 experienced serious adverse events (pyrexia, hemorrhage or events relating to the patient’s underlying protein C deficiency)
- The common adverse reactions observed in clinical trials were rash, itching and lightheadedness.1
See additional Important Safety Information about CEPROTIN
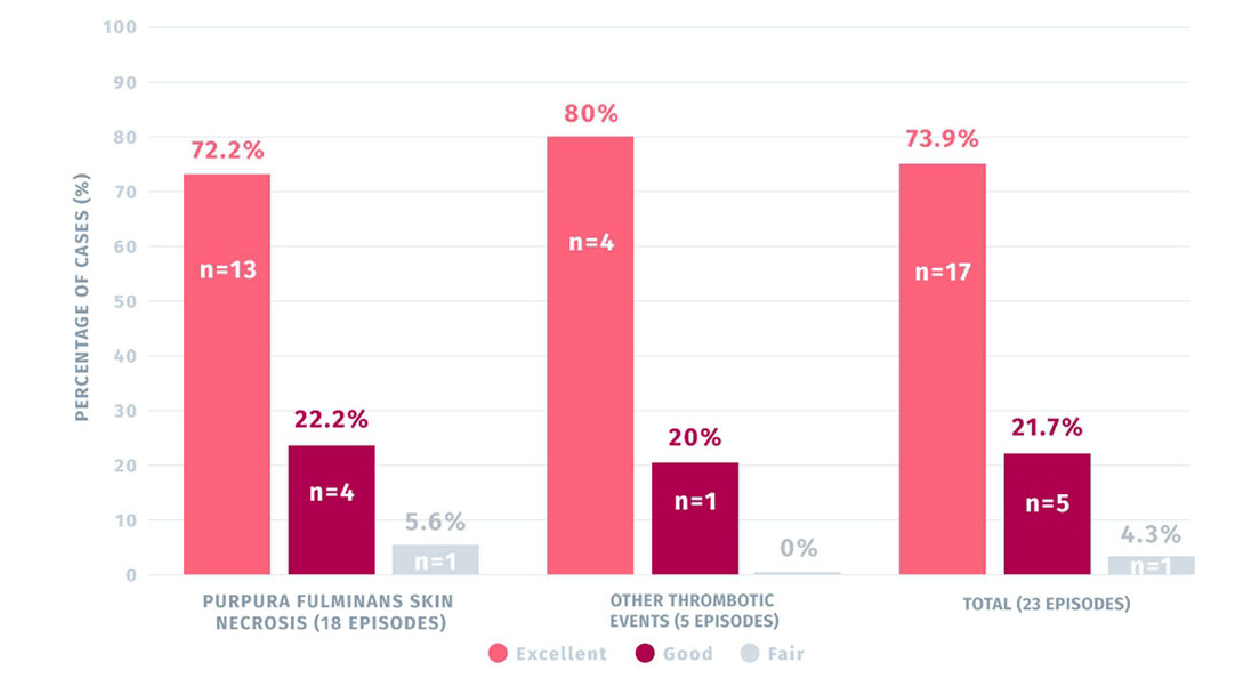
72.2% OF PURPURA FULMINANS SKIN NECROSIS AND 80% OF OTHER THROMBOTIC EVENTS TREATED WITH CEPROTIN WERE RATED EXCELLENT1
Excellent:2
- The extent of thrombus at day 2–3 < baseline and no extension of thrombus after day 2–3 while on protein C concentrate until successful establishment of adequate anti-coagulation
Good:2
- The extent of thrombus at day 4–5 < day 2–3 and no extension of thrombus after day 4–5 while on protein C concentrate until successful establishment of adequate anti-coagulation
Fair:2
- No extension of thrombus after day 4–5 while on protein C concentrate until successful establishment of adequate anti-coagulation
Secondary efficacy rating of CEPROTIN for the treatment of PF skin lesions (n=18 episodes)
and other thrombotic events (n=5 episodes)1
- 72,2% of the cases of skin lesions due to purpura fulminans treated with CEPROTIN were rated as excellent1
- 80% of cases of other thrombotic events treated with CEPROTIN were rated excellent1
Please click for information on CEPROTIN dosing
Warnings and Precautions
Hypersensivity: CEPROTIN may contain trace amounts of mouse protein and/or heparin as a result of the manufacturing process. Allergic reactions to mouse protein and/or heparin cannot be ruled out. If symptoms of hypersensitivity/allergic reaction occur, discontinue the injection/infusion. In case of anaphylactic shock, the current medical standards for treatment are to be observed.
Transmission of infectious agents: Because CEPROTIN is made from human plasma, it may carry a risk of transmitting infectious agents, e.g. viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and theoretically, the Creutzfeld-Jakob disease (CJD) agent.
Bleeding episodes: Several bleeding episodes have been observed in clinical studies. Concurrent anticoagulant medication may have been responsible for these bleeding episodes. However, it cannot be completely ruled out that the administration of CEPROTIN further contributed to these bleeding events. Simultaneous administration of CEPROTIN and tissue plasminogen activator (tPA) may further increase the risk of bleeding from tPA.
Heparin-induced thrombocytopenia (HIT): CEPROTIN contains trace amounts of heparin which may lead to HIT, which can be associated with a rapid decrease of the number of thombocytes. If HIT is suspected, determine the platelet count immediately and consider discontinuation of CEPROTIN.
Low sodium diet/Renal impairment: Patients on a low sodium diet or who have renal impairment should be informed that the quantity of sodium in the maximum daily dose of CEPROTIN exceeds 200 mg. Monitor patients with renal impairment closely for sodium overload.
Adverse Reactions
Common adverse reactions related to CEPROTIN observed in clinical trials were hypersensitivity or allergic reactions: lightheadedness, itching and rash.
INDICATION
CEPROTIN [Protein C Concentrate (Human)] is indicated for neonates, pediatric and adult patients with severe congenital Protein C deficiency for the prevention and treatment of venous thrombosis and purpura fulminans.
Please click for Full Prescribing Information.
References:
-
CEPROTIN [Protein C Concentrate (Human)] Prescribing information. Lexington, MA: Baxalta US Inc.
-
Manco-Johnson MJ, et al. Efficacy and safety of protein C concentrate to treat purpura fulminans and thromboembolic events in severe congenital protein C deficiency. Thromb Haemost. 2016;116:56-68.